Abstract
BACKGROUND: Blood or marrow transplant (BMT) recipients are at increased risk of accelerated atherosclerosis due to prior exposure to chemotherapy and radiation, and pre-existing/new onset comorbidities. This increases the risk of long-term cardiovascular disease (CVD) including coronary heart disease and stroke. There is a need to identify BMT recipients at highest risk of CVD to develop targeted intervention strategies. We performed a comprehensive evaluation of the risk of late-occurring CVD in BMT survivors and developed a prediction model using the resources offered by BMT Survivor Study (BMTSS).
METHODS: BMTSS includes patients who received BMT at one of the three participating US sites between 1974 and 2014, survived ≥2 years after BMT and completed the BMTSS survey. The survey asked participants to report specific chronic health conditions diagnosed by their healthcare provider (including coronary heart disease and stroke as well as cardiovascular risk factors [CVRFs: hypertension, diabetes and dyslipidemia]), along with age at diagnosis. They also provided information on socio-demographics (sex, race/ethnicity, education, income) and health behaviors such as smoking and alcohol intake. Medical records were abstracted for information regarding primary cancer diagnosis, therapeutic exposures (pre-BMT chemotherapy/radiation, transplant conditioning regimens), stem cell source (autologous, allogeneic), graft type (bone marrow, cord blood or peripheral blood stem cells), and history of chronic graft vs. host disease (among allogeneic BMT recipients). All participants provided informed consent.
Sixty percent of the cohort was used for discovery and the remainder for replication of the risk model. Cox regression models estimated the model's discrimination based on the concordance (C) statistic. Variables selected in our CVD risk model included: age at BMT, sex, race/ethnicity, chest/ neck/ cranial radiation, health behaviors and CVRFs present at BMT.
RESULTS: The study included 3,848 BMT survivors; 53.4% had received an allogeneic BMT; 55.9% were males; 75.2% were non-Hispanic whites. Median age at study participation was 56.3 years (interquartile range: 41.3-65.5y); median follow up from BMT was 9.1 years (interquartile range: 5.9-15.3y). CVD was diagnosed after BMT in 238 participants (117 allogeneic; 121 autologous). Conditional on surviving ≥2 years after BMT, the cumulative incidence of CVD was 5.8% at 10 years (allogeneic BMT: 5.0%; autologous BMT: 6.8%) and 9.8% at 20 years (allogeneic BMT: 7.8%; autologous BMT: 13.2%).
Risk factors for post-BMT CVD: Increasing age at BMT (hazard ratio [HR]=1.03/y, 95% confidence interval [CI]: 1.02-1.04, p<0.0001), male sex (HR=1.50, 95%CI: 1.13-1.94, p=0.005), history of smoking (HR=1.54, 95%CI:1.08-2.20, p=0.02), hypertension at BMT (HR=1.68, 95%CI: 1.27-2.22, p=0.0003), dyslipidemia at BMT (HR=1.66, 95%CI: 1.24-2.22, p=0.0006), chest radiation (HR=1.86, 95%CI: 1.06-3.24, p=0.029) and neck/cranial radiation (HR=1.62, 95%CI: 0.99-2.67, p=0.057) were associated with increased CVD risk in multivariable analysis.
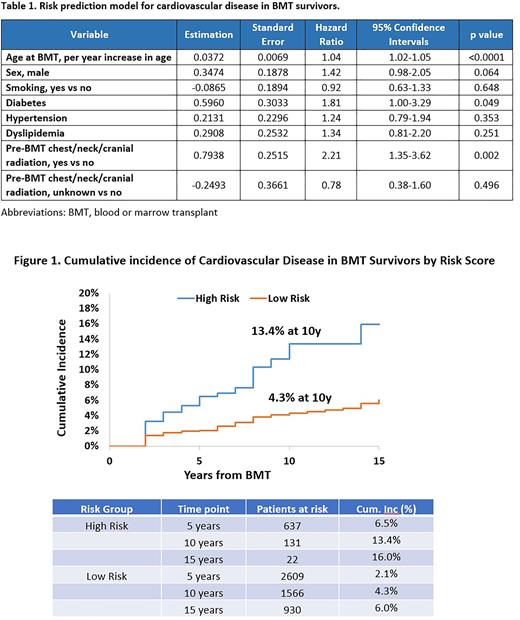
Risk prediction model for CVD in BMT survivors: Inclusion of age at BMT, sex, history of smoking, diabetes, hypertension or dyslipidemia at BMT, and pre-BMT chest/neck/cranial radiation yielded C statistics of 0.69 (95%CI: 0.63-0.75) in the training and 0.67 in the testing set (95%CI: 0.61-0.73) (Table 1). Risk scores were derived by adding parameter estimates of each variable, and a cut point was determined to maximize the C statistics. Survivors were then assigned to statistically distinct risk groups corresponding to cumulative incidence of CVD of 13.4% in high-risk group (risk score >1) vs. 4.3% in the low-risk group (score ≤1) at 10 years post-BMT (Figure 1).
CONCLUSION: Traditional CVRFs at the time of BMT as well as history of chest, neck and cranial radiation can be combined to identify BMT survivors at high risk for CVD. The risk prediction model provides a framework for future targeted screening and interventions.
Disclosures
Gangaraju:Alexion: Consultancy; Sanofi Genzyme: Consultancy. Landier:Merck Sharp & Dohme: Other: Wendy Landier's institution has received vaccine supply and laboratory services for an unrelated clinical trial through the Investigator Initiated Studies Program of Merck Sharp & Dohme (MISP #40083). Weisdorf:FATE Therapeutics: Other: Research Support; Incyte: Other: Research support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal